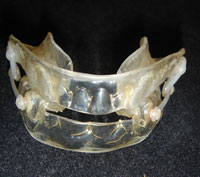
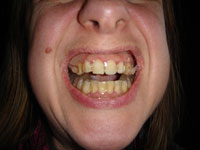
The mandibular protraction orthosis is a highly effective treatment for snoring and apnea syndromes.
The mandibular protraction orthosis is the only "reasonable" treatment for excess tongue volume or retrognathia in adults. It involves fitting an appliance that holds the mandible forward during sleep, while at the same time advancing the base of the tongue and giving it more room.
The mechanics of mandibular advancement work on two levels:
- Increased upper airway caliber
- Reduces the risk of upper airway closure by tensioning the pharyngeal wall
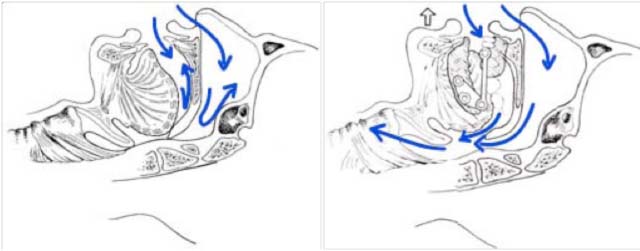
mechanism of action of orthotics
This treatment is currently very popular because it's simple and highly effective, but it's essential to be wary of the large number of orthotic models on sale in pharmacies or on the Internet. To be effective, braces must be custom-made after a dental check-up.
- Tailor-made because it is thermoformed, it can be mobilized at night, and propulsion is not adapted to the patient's needs. At present, only the ORM mandibular protraction orthosis from RESMED / NARVAL laboratories is covered by social security under certain conditions.
- After a dental check-up, as the tensile forces are considerable, it is important to check for tooth mobility, periodontal disease and the strength of the remaining teeth.
- A panoramic dental X-ray is often useful
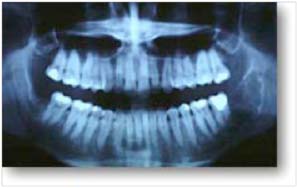
WHAT TYPE OF ORTHOSIS SHOULD BE CONSIDERED?
Consensus in favor of custom-made bibloc orthoses
- They are scientifically validated and recognized by the HAS (haute autoritée de santé).
- Custom orthotics are more effective and better tolerated,
- one-piece orthoses are used less and less frequently due to their lower tolerance; two-piece orthoses allow vertical and lateral movement and offer an advantage in terms of lower risk of joint complications
temporo-mandibular
Only custom-made bibloc orthoses made by RESMED / NARVAL laboratories are recognized by the HAS and are eligible for reimbursement under certain conditions, as specified in the official journal of October 31, 2008.
LPP Code : 2497884 Designation : ORTHESE D'AVANCEE MANDIBULAIRE, NARVAL, O.R.M Orthoses and external prostheses. Non-orthopedic external prostheses. Orthèse d'avance mandibulaire O.R.M., Laboratoires Narval SA. Coverage of the O.R.M. mandibular advancement orthosis is provided for the treatment of severe SAHOS (apnea/hypopnea index AHI greater than 30 or, less than or equal to 30 and greater than or equal to 5 associated with severe daytime sleepiness) as a second-line treatment after refusal or intolerance of continuous positive airway pressure (CPAP). Coverage is provided on the basis of a prior agreement filled in by the prescribing physician at the time of the first prescription and at each renewal. The social security organization's response must be sent within the timeframe specified in article R. 16523 of the French Social Security Code. The O.R.M. mandibular advancement orthosis is a custom-made device. Prescribing this orthosis requires collaboration between a sleep specialist (diagnosis, treatment, follow-up) and a practitioner with knowledge of both sleep and the manducatory apparatus (dental examination, impressions, adjustments and settings): - the diagnosis of sleep apnea must be documented by a clinical and polysomnographic examination (or by ventilatory polygraphy); - the prescription must be preceded by a dental examination to rule out any dental or articular contraindications. Ventilatory polygraphy or polysomnography should be used to check the effectiveness of the orthosis. Rigorous long-term monitoring by a sleep specialist is essential. The manducatory apparatus must be monitored every 6 months. Coverage of the M.R.O. orthosis excludes the possibility of treatment with continuous positive airway pressure (CPAP). In the event of proven failure of orthotic treatment, CPAP may nevertheless be proposed. The orthosis is guaranteed for 1 year. Renewal is only authorized after 2 years have elapsed since the previous fitting, and is subject to : - demonstration of efficacy (improvement in symptoms and at least 50% reduction in AHI on control polysomnography under M.R.O.); - compliance with odontological follow-up. The prescriber will need to justify any early renewal. Prior agreement: Yes
Dates J.O. and Arrêté 31/10/2008 28/
ARE THERE ANY SIDE EFFECTS TO WEARING ORTHOTICS?
- Short-term side effects are frequent but minor and generally transient (disappearing 10-30' after waking up).
- Dental and gum pain
- Joint numbness and sensation of loss of bite
- Hypersalivation
- Dry mouth
- The risk of orthodontic side-effects is low, especially when the advancement does not exceed 6mm
- The main long-term risk (5+ years) is incisor versioning due to the anteroposterior forces exerted by the splint.
- no triggering of temporomandibular joint pathology was observed, but rather an improvement, with the orthosis acting as a "stretching" effect and preventing teeth grinding (bruxism)
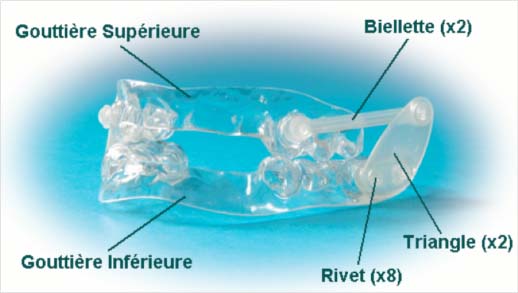
ORM traction orthesis NARVAL
FOLLOW-UP TO ORTHOTIC TREATMENT
- In the case of sleep apnea, the effectiveness of the orthosis must be monitored by nocturnal polygraphy.
- Adaptation of mandibular propulsion by changing bielet size according to tolerance and efficiency is often necessary.
- In case of regular use, the orthosis must be renewed after an average of 2 years.